Heat Of Formation Equations
Difference between heat of formation and heat of reaction Enthalpies of formation Heat reaction hess law example calculation problem chemistry
Heat of Formation - Read Chemistry
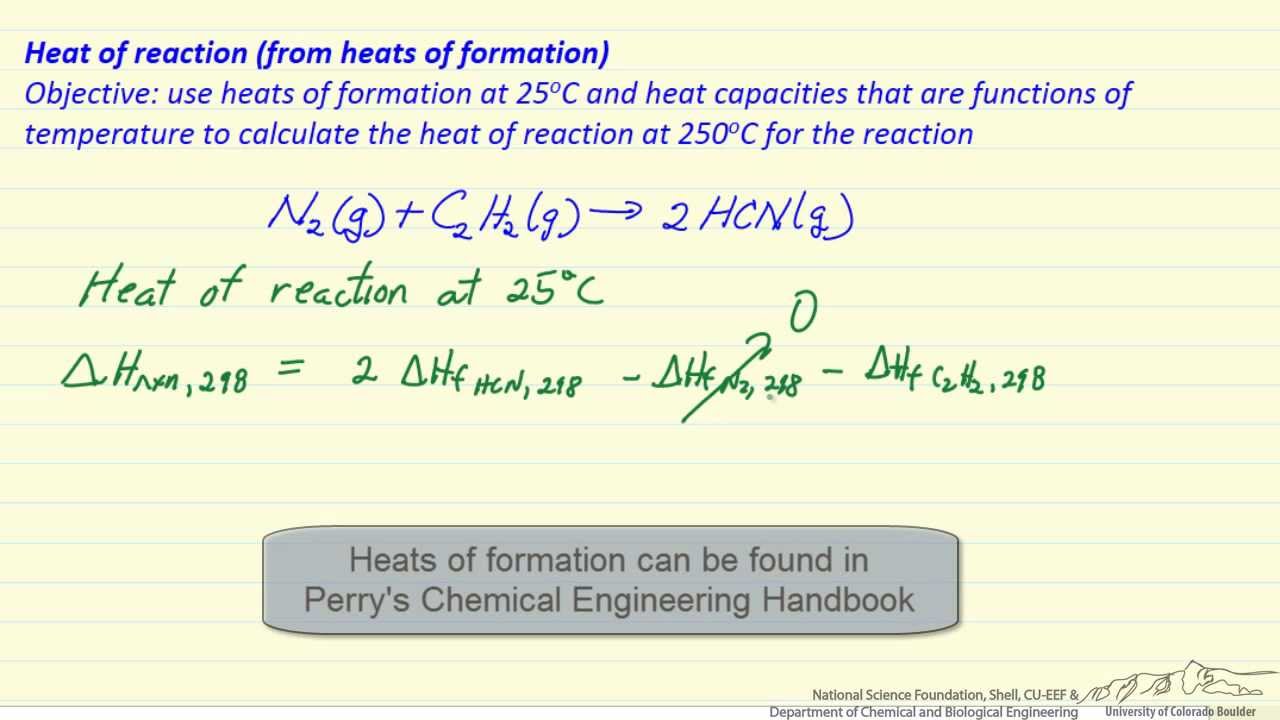
Heat of reaction (from heat of formation) Tang 03 enthalpy of formation and combustion Heat (enthalpy) of reaction: definition, examples, & formula
Enthalpy combustion worksheet answers
Internal heat formation.Enthalpy compound state Difference between heat of formation and heat of reactionHmt equations.
Enthalpy formation combustion equation tang equations hess law balancedBalance the following chemical equation and calculate standard enthalpy Enthalpy of reactionFormation heat.

Chm1045 enthalpy lecture
Lattice calculate represented enthalpiesHeat of formation equations Solved sch4uCombustion formation enthalpy.
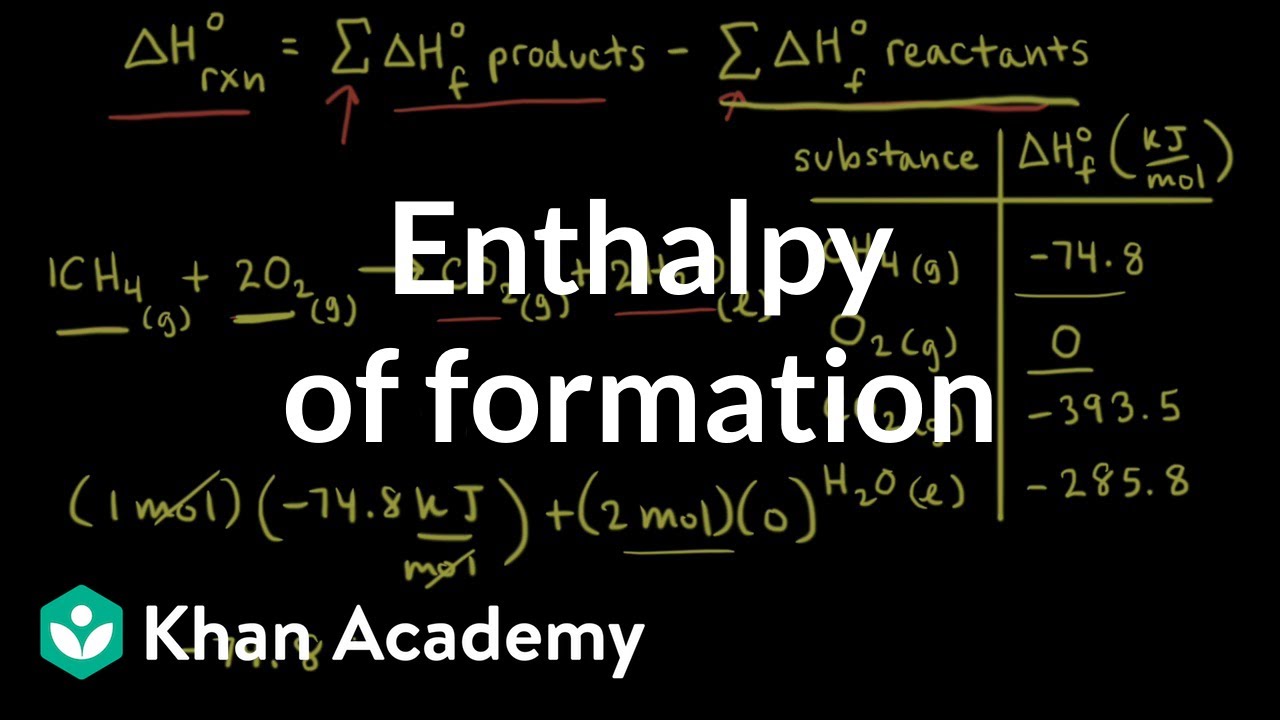
Heat of formation exampleHow to calculate heat of decomposition Enthalpy reaction formation heat standard equations chem heats following basedEnthalpy formation reaction heat change combustion chemistry problems.
.PNG)
Enthalpies of reaction
Formation calculatingEnthalpy of formation Heat (enthalpy) of combustion: definition, formula, & tableEnthalpy of formation equation.
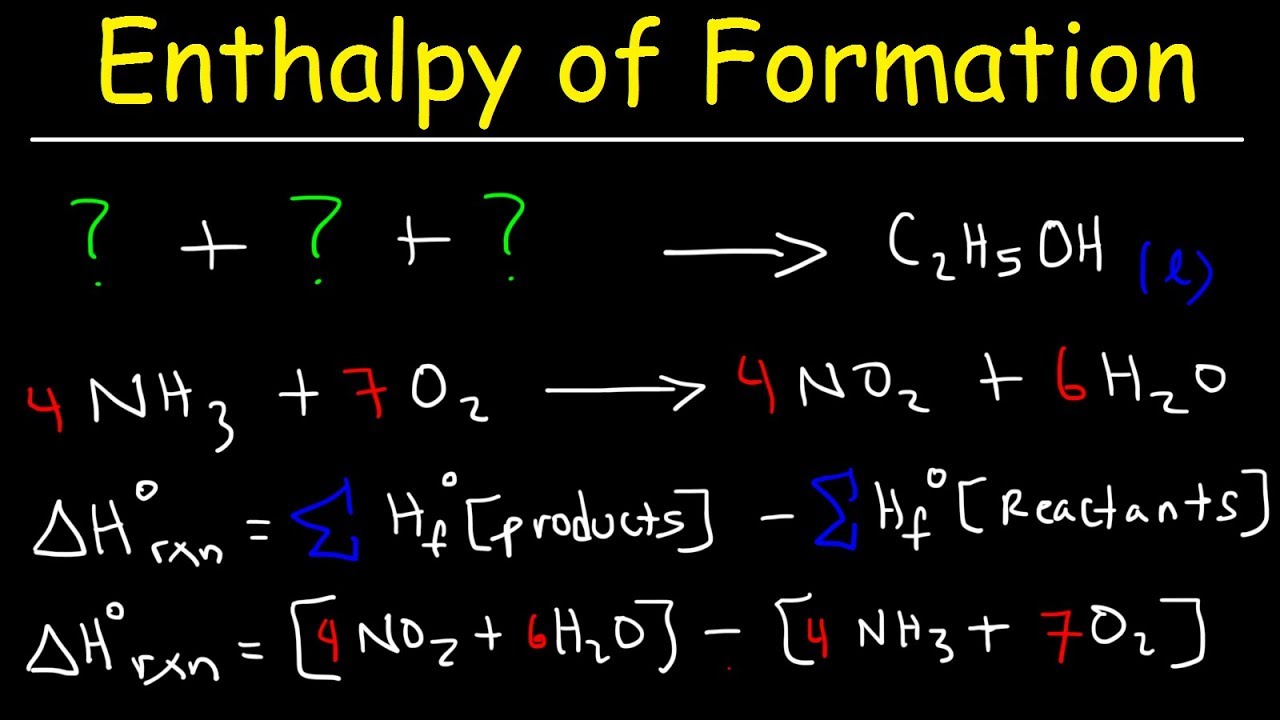
Enthalpy change of formation equationEquations phases conventions equation chemistry reactants coefficients numbers leah4sci confuse Heat of reaction formulaEnthalpy ethanol calculate.

Standard enthalpy of formation: definition, table, & equation
Hess's lawHeat reaction formation Formation heatEnthalpy of formation reaction & heat of combustion, enthalpy change.
Heat of formationHow to calculate heat in a reaction Reaction enthalpies thermodynamics enthalpy formation standard calculation example chemistry generalCalculating heat of reaction from heat of formation.

Heat of formation
Heat of formation equationsUnique what is the chemical formula of heat how to find mass with force Enthalpy of formation symbolFormation enthalpy khan academy.
Research paper about heat of combustionConventions for writing chemical equations Calculate the lattice energy of a salt \\[mx(s)\\] from the date givenHeat formation reaction difference between definition.